Part two of a four-part series about an athlete’s journey to save his leg.
(In part one, JH, age 62, was hit in the shin by a baseball. A hematoma developed that became infected; treatment included opening and draining the hematoma and discharge to home to self-administer IV antibiotics through a PICC line.)
Sitting on the sofa in his den the day after discharge, JH had his leg propped up. Looking at the wide-open wound, he knew something wasn’t right. With waning confidence in the situation, JH thought the injury looked worse after the treatment to drain it than it did before the hospital visit.
Though JH and his wife had videotaped the home care nurse’s instructions to administer the IV medication, the effort did not go well. Unable to make the tubes connect, blood began pouring from the PICC line, saturating his clothing. In the midst of this, his physician friend responded to the photos. He told JH to go immediately to the hospital, not to stop for breakfast, not to change clothes – but to go immediately.
Having gotten the PICC line capped, JH took an Uber to a Philadelphia medical center. After a long wait in the ED, JH was admitted, evaluated, and quickly scheduled for surgery to debride the wound. In surgery, tissue from the shin was debrided to the bone. The wound was covered but did not begin healing the way his care team had hoped.
Three days after the first surgery, a second surgery was required for additional debridement. This time a vacuum drain was inserted into the area and sealed with a clear wrap with suction applied to the wound. Lab cultures returned positive for Staphylococci. JH began to comprehend more fully the conversations with the physicians about the infection spreading more seriously into the bones in his leg and the growing concern that amputation might be required to prevent systemic infection or even death.
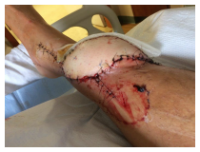 JH is part of a well-connected long-time Main Line Philadelphia family. Those connections enabled him to be placed in the care of a renowned physician known most recently for successful pediatric hand transplants. JH was evaluated and a course of action was determined. In a third surgical procedure, a full-thickness flap, with vasculature, was harvested along with a graft of skin shaved from his thigh, all transplanted to the lower leg. The purpose was to create a “biologic dressing”—the patient’s skin, that would protect against bacterial contamination and hasten to heal.
JH is part of a well-connected long-time Main Line Philadelphia family. Those connections enabled him to be placed in the care of a renowned physician known most recently for successful pediatric hand transplants. JH was evaluated and a course of action was determined. In a third surgical procedure, a full-thickness flap, with vasculature, was harvested along with a graft of skin shaved from his thigh, all transplanted to the lower leg. The purpose was to create a “biologic dressing”—the patient’s skin, that would protect against bacterial contamination and hasten to heal.
After seven hours of surgery, JH awoke in the ICU. In the event of flap failure, JH needed to remain under close watch. The care team monitored the pulse in the surgical area.
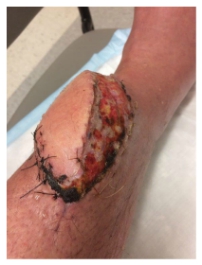 The flap was an ecosystem – a living environment around the initial bruise site that supported tissue repair and healing and covered that bone. JH called it a “hoagie roll” – a protrusion on his lower leg that extended about six inches along a left to right down angle across his leg. The plan seemed to be working. The swelling decreased, the infection seemed to be clearing. For the first time, the wound was sutured closed and wrapped. Discharge care included topical antibiotic ointment for the sutures and instructions to keep the wound covered. The pain was gone.
The flap was an ecosystem – a living environment around the initial bruise site that supported tissue repair and healing and covered that bone. JH called it a “hoagie roll” – a protrusion on his lower leg that extended about six inches along a left to right down angle across his leg. The plan seemed to be working. The swelling decreased, the infection seemed to be clearing. For the first time, the wound was sutured closed and wrapped. Discharge care included topical antibiotic ointment for the sutures and instructions to keep the wound covered. The pain was gone.
For the next 10 days, JH healed. Back at work with the wound covered by the “hoagie roll,” he was on a trajectory toward full recovery. With sutures out and butterfly bandages along the repair line, JH was confident he would soon return to the baseball diamond. Instead, he was to return to surgery for a fourth, fifth and sixth time. He and his leg remained in danger.
(In part three, JH faces emergency surgery, a maggot infiltration and the specter of a polymicrobial infection. His game is far from over and a win, even with the best players and tools, is not a sure thing.)
About the Author
Dr. Pollack is certified in Emergency Medicine and is a founding board member of the Hospital Quality Foundation. Visit: www.hospitalqualityfoundation.org.

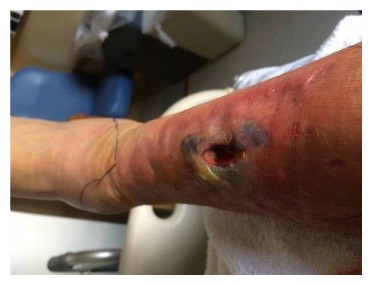 Contusions caused by impact rarely become infected when there is no break in the skin. However, staphylococcus and streptococcus are the most common types of bacteria to enter a skin break. Contusion’s pool of blood becomes food for the bacteria. It can ordinarily be treated with oral antibiotics [
Contusions caused by impact rarely become infected when there is no break in the skin. However, staphylococcus and streptococcus are the most common types of bacteria to enter a skin break. Contusion’s pool of blood becomes food for the bacteria. It can ordinarily be treated with oral antibiotics [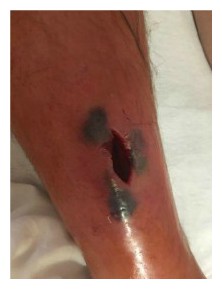 While the treatment seemed logical and appropriate to him, the pain after inactivity returned with great intensity. After two days, he was released. The wound was open, deep nearly to the bone, and left uncovered with no bandage or covering at all. Discharge instructions included pouring hydrogen peroxide into the wound, self-administration of antibiotic fluids twice per day and care of a PICC line by which the IV fluids would be dripped.
While the treatment seemed logical and appropriate to him, the pain after inactivity returned with great intensity. After two days, he was released. The wound was open, deep nearly to the bone, and left uncovered with no bandage or covering at all. Discharge instructions included pouring hydrogen peroxide into the wound, self-administration of antibiotic fluids twice per day and care of a PICC line by which the IV fluids would be dripped.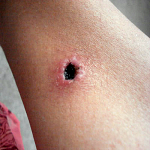 Enterocutaneous (ECF) and entero-atmospheric fistulas (EAF) can create a challenge for the certified wound clinician. The hope is always that the fistula will spontaneously close while at the same time managing the current situation.
Enterocutaneous (ECF) and entero-atmospheric fistulas (EAF) can create a challenge for the certified wound clinician. The hope is always that the fistula will spontaneously close while at the same time managing the current situation.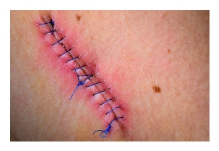 Surgical site infections (SSI) increase medical costs, length of hospital stays, and readmission rates. Although this rate may be under-reported, the incidence of SSI in the US is estimated to be 2.8%. In the inpt setting or generally?
Surgical site infections (SSI) increase medical costs, length of hospital stays, and readmission rates. Although this rate may be under-reported, the incidence of SSI in the US is estimated to be 2.8%. In the inpt setting or generally? As a talented group of professionals in our areas of specialization, we have formed a common goal to conquer any wound, regardless of the location, source, chronicity, or barriers to healing. At least, this may be our belief. Did any of us have a burning desire as aspiring professionals to work to advance the cause of evidence-based wound care? As a team of multidisciplinary professionals of physicians, nurses, pharmacists, physical therapists, researchers, and industry personnel, we were able to recognize an area of need for patients who suffer from chronic wounds. For each of us, there was something which ignited the passion to serve, to research, and to lend our expertise to this subject. Our strides to promote wound care from a multidisciplinary approach have led us to improved overall outcomes for our patients.
As a talented group of professionals in our areas of specialization, we have formed a common goal to conquer any wound, regardless of the location, source, chronicity, or barriers to healing. At least, this may be our belief. Did any of us have a burning desire as aspiring professionals to work to advance the cause of evidence-based wound care? As a team of multidisciplinary professionals of physicians, nurses, pharmacists, physical therapists, researchers, and industry personnel, we were able to recognize an area of need for patients who suffer from chronic wounds. For each of us, there was something which ignited the passion to serve, to research, and to lend our expertise to this subject. Our strides to promote wound care from a multidisciplinary approach have led us to improved overall outcomes for our patients. Despite the vast amount of advanced wound care products available as well as an evidence-based practice that supports wet to dry dressings are substandard, I still receive daily calls from clinicians reporting new wound care orders for wet to dry dressings to be performed in the home setting, usually twice daily.
Despite the vast amount of advanced wound care products available as well as an evidence-based practice that supports wet to dry dressings are substandard, I still receive daily calls from clinicians reporting new wound care orders for wet to dry dressings to be performed in the home setting, usually twice daily. I am that person, the one who is thrilled the more challenging the wound looks. But I guess we all are, right? That is what has brought us together, this group of people who have never met, we are all connected through one simple thing, a passion for those hard to heal wounds. Unfortunately, we likely all have had those wounds that we have tried hard to heal and failed. That’s right, I have failed, we all have. I wish I was perfect; I wish I got it right from the start every time, but I don’t. It’s these really difficult wounds that help us grow as practitioners and I hope that as I share my mistakes with you, you don’t have to make them as well.
I am that person, the one who is thrilled the more challenging the wound looks. But I guess we all are, right? That is what has brought us together, this group of people who have never met, we are all connected through one simple thing, a passion for those hard to heal wounds. Unfortunately, we likely all have had those wounds that we have tried hard to heal and failed. That’s right, I have failed, we all have. I wish I was perfect; I wish I got it right from the start every time, but I don’t. It’s these really difficult wounds that help us grow as practitioners and I hope that as I share my mistakes with you, you don’t have to make them as well. Members of AAWC are known for top tier commitment, specialization, and passion for wound care. Yet among the greatest of challenges advanced wound care professionals face, one is how to demonstrate and communicate the impact and value provided to administrators and clinicians managing or overseeing multiple clinical services.
Members of AAWC are known for top tier commitment, specialization, and passion for wound care. Yet among the greatest of challenges advanced wound care professionals face, one is how to demonstrate and communicate the impact and value provided to administrators and clinicians managing or overseeing multiple clinical services.

 doesn’t have all the tools, then the solution relies on creativity. Hands-on sessions encouraged attendees to be creative and get in the habit of choosing the best course of action given select resources when practicing wound care.
doesn’t have all the tools, then the solution relies on creativity. Hands-on sessions encouraged attendees to be creative and get in the habit of choosing the best course of action given select resources when practicing wound care. 
