Part three of a four-part series about an athlete’s fight to beat a leg infection and save his leg. In parts one and two, JH, age 62, was hit in the shin by a baseball. Treatment included three surgeries to open and drain the contusion, another to remove necrotic tissue and a third to graft a vein, artery and skin from his thigh to the wound. Nearly healed, JH was preparing to get back to baseball when a curve ball came his way.)
Managing a busy life with what JH called a hoagie roll on his lower leg, presented its own set of challenges. The hoagie roll created an environment to feed the wound area with blood and enable it to heal, covering what had been exposed bone with viable tissue. Despite the new appendage, he could see the end game, with full healing on the horizon and with a return to the routine of family, work, and sports. Sutures out and the wound healing, the hoagie roll was unattractive and in the way of socks and trouser legs, but it was to be surgically reduced in a few weeks.
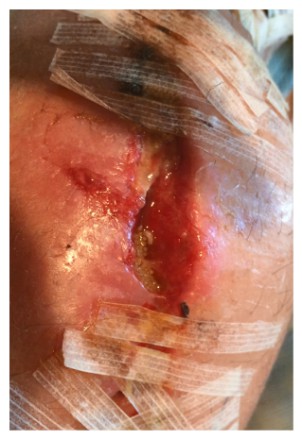
After a morning shower 10 days after returning home, JH noticed some orange-tinted, clear fluid oozing from the incision line. Peeling back one of the many butterfly bandages to take a look, the wound opened up along the incision line, releasing more fluid. In the wound, he saw something moving. Then he noticed a handful of somethings disappearing into the wound and returning to the surface again and again. Maggots and hundreds of small, oval white maggot eggs filled the wound.
JH sent a picture while simultaneously calling his physician. In denial, his physician said, “It can’t be.” A Friday, JH was to see the doctor the following Tuesday. He was told to wipe the incision line clean and keep the Tuesday appointment. But, the doctor said, it would also be fine for him to go to an emergency department for evaluation if he would be more comfortable with that.
Horrified both with what he was seeing on his leg and what he was hearing from his doctor, JH headed back to Philadelphia.
On examination, the physician assistant exclaimed, “There are maggots in there!” For the first time in weeks, JH expressed dismay with the resident and became animated – an understatement at best, belying weeks of frustration. He shouted, “I told you – and you wanted me to wait five days to be seen?”
When it occurs, maggot infestation, known as Myiasis, is considered to represent a breakdown in standards of care. As a result, there are few statistics on the frequency of these occurrences [Myiasis: maggot infestation]. Myiasis typically results when a common female housefly lands on a wound or wound dressing to feed and lays eggs there. One fly can lay between 50-300 eggs at one time. The eggs hatch within 12 hours and are fully grown within 60 hours. Shortly thereafter, they migrate to drier feeding territory.
 While conceptually a thoroughly disgusting situation, maggots have been historically used to facilitate wound healing. Prior to antibiotics, maggots were intentionally introduced to some wounds and were found to promote healing by eating only necrotic—not viable—tissue, increasing exudate, digesting some forms of bacteria and secreting certain enzymes to break down necrotic tissue [Myiasis: maggot infestation].
While conceptually a thoroughly disgusting situation, maggots have been historically used to facilitate wound healing. Prior to antibiotics, maggots were intentionally introduced to some wounds and were found to promote healing by eating only necrotic—not viable—tissue, increasing exudate, digesting some forms of bacteria and secreting certain enzymes to break down necrotic tissue [Myiasis: maggot infestation].
While myiasis would resolve itself within weeks as maggots seek other environments, both patients and caregivers typically find the wait intolerable and removal is necessary. In JH’s case, waiting was not a consideration [Myiasis: maggot infestation].
Immediately rushed back into surgery for the fourth time, the surgeons tried to clean the wound of infection again, and to remove necrotic tissue, maggots, and larvae. Remarkably, there was still no infection in the bones, but new tissue cultures revealed the infection was now caused primarily by E. coli; it’s unclear whether this was a polymicrobial infection from the start, or if the prior antibiotic therapy had been effective against Staphylococcus and now a new infection had been introduced. Common in the digestive tracts of healthy individuals, E. coli in an infection can typically be treated with antibiotics.
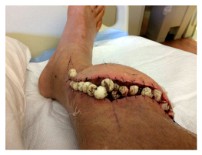 During this latest surgery, twenty antibiotic beads were placed into the wound. Antibiotic beads were developed for use with joint replacement surgery to reduce the potential for infection in patients undergoing hip replacements [Tiny antibiotic beads fight infections after joint replacement]. The beads release antibiotics slowly over a period of up to six weeks. JH’s wound remained undressed for three days while it was watched closely for indications of healing from the deep parts of the wound towards the skin, and, of course, for signs of a new infection.
During this latest surgery, twenty antibiotic beads were placed into the wound. Antibiotic beads were developed for use with joint replacement surgery to reduce the potential for infection in patients undergoing hip replacements [Tiny antibiotic beads fight infections after joint replacement]. The beads release antibiotics slowly over a period of up to six weeks. JH’s wound remained undressed for three days while it was watched closely for indications of healing from the deep parts of the wound towards the skin, and, of course, for signs of a new infection.
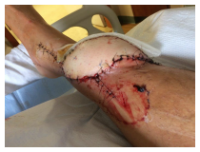
A fifth surgery later closed the wound. JH’s leg was cast, and he was sent home with instructions to return in 10 days to remove both the cast and sutures. The cast was an ultraconservative measure intended to prevent any chance of additional infection or infestation.
(In the final segment of this series, JH will face additional surgeries and learn whether he will ever return to his busy and athletic life or whether the infection will enter his bone and bloodstream.)

 JH is part of a well-connected long-time Main Line Philadelphia family. Those connections enabled him to be placed in the care of a renowned physician known most recently for successful pediatric hand transplants. JH was evaluated and a course of action was determined. In a third surgical procedure, a full-thickness flap, with vasculature, was harvested along with a graft of skin shaved from his thigh, all transplanted to the lower leg. The purpose was to create a “biologic dressing”—the patient’s skin, that would protect against bacterial contamination and hasten to heal.
JH is part of a well-connected long-time Main Line Philadelphia family. Those connections enabled him to be placed in the care of a renowned physician known most recently for successful pediatric hand transplants. JH was evaluated and a course of action was determined. In a third surgical procedure, a full-thickness flap, with vasculature, was harvested along with a graft of skin shaved from his thigh, all transplanted to the lower leg. The purpose was to create a “biologic dressing”—the patient’s skin, that would protect against bacterial contamination and hasten to heal.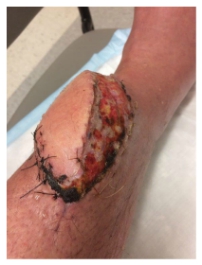 The flap was an ecosystem – a living environment around the initial bruise site that supported tissue repair and healing and covered that bone. JH called it a “hoagie roll” – a protrusion on his lower leg that extended about six inches along a left to right down angle across his leg. The plan seemed to be working. The swelling decreased, the infection seemed to be clearing. For the first time, the wound was sutured closed and wrapped. Discharge care included topical antibiotic ointment for the sutures and instructions to keep the wound covered. The pain was gone.
The flap was an ecosystem – a living environment around the initial bruise site that supported tissue repair and healing and covered that bone. JH called it a “hoagie roll” – a protrusion on his lower leg that extended about six inches along a left to right down angle across his leg. The plan seemed to be working. The swelling decreased, the infection seemed to be clearing. For the first time, the wound was sutured closed and wrapped. Discharge care included topical antibiotic ointment for the sutures and instructions to keep the wound covered. The pain was gone. 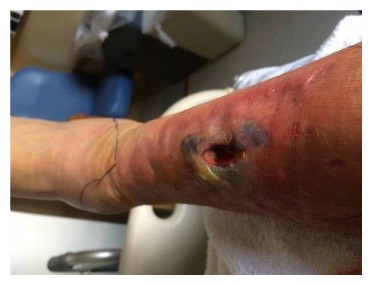 Contusions caused by impact rarely become infected when there is no break in the skin. However, staphylococcus and streptococcus are the most common types of bacteria to enter a skin break. Contusion’s pool of blood becomes food for the bacteria. It can ordinarily be treated with oral antibiotics [
Contusions caused by impact rarely become infected when there is no break in the skin. However, staphylococcus and streptococcus are the most common types of bacteria to enter a skin break. Contusion’s pool of blood becomes food for the bacteria. It can ordinarily be treated with oral antibiotics [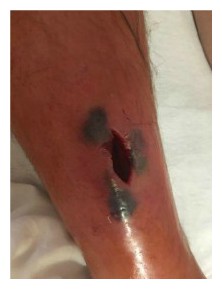 While the treatment seemed logical and appropriate to him, the pain after inactivity returned with great intensity. After two days, he was released. The wound was open, deep nearly to the bone, and left uncovered with no bandage or covering at all. Discharge instructions included pouring hydrogen peroxide into the wound, self-administration of antibiotic fluids twice per day and care of a PICC line by which the IV fluids would be dripped.
While the treatment seemed logical and appropriate to him, the pain after inactivity returned with great intensity. After two days, he was released. The wound was open, deep nearly to the bone, and left uncovered with no bandage or covering at all. Discharge instructions included pouring hydrogen peroxide into the wound, self-administration of antibiotic fluids twice per day and care of a PICC line by which the IV fluids would be dripped.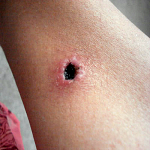 Enterocutaneous (ECF) and entero-atmospheric fistulas (EAF) can create a challenge for the certified wound clinician. The hope is always that the fistula will spontaneously close while at the same time managing the current situation.
Enterocutaneous (ECF) and entero-atmospheric fistulas (EAF) can create a challenge for the certified wound clinician. The hope is always that the fistula will spontaneously close while at the same time managing the current situation.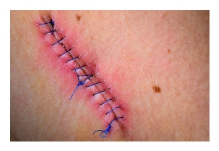 Surgical site infections (SSI) increase medical costs, length of hospital stays, and readmission rates. Although this rate may be under-reported, the incidence of SSI in the US is estimated to be 2.8%. In the inpt setting or generally?
Surgical site infections (SSI) increase medical costs, length of hospital stays, and readmission rates. Although this rate may be under-reported, the incidence of SSI in the US is estimated to be 2.8%. In the inpt setting or generally? As a talented group of professionals in our areas of specialization, we have formed a common goal to conquer any wound, regardless of the location, source, chronicity, or barriers to healing. At least, this may be our belief. Did any of us have a burning desire as aspiring professionals to work to advance the cause of evidence-based wound care? As a team of multidisciplinary professionals of physicians, nurses, pharmacists, physical therapists, researchers, and industry personnel, we were able to recognize an area of need for patients who suffer from chronic wounds. For each of us, there was something which ignited the passion to serve, to research, and to lend our expertise to this subject. Our strides to promote wound care from a multidisciplinary approach have led us to improved overall outcomes for our patients.
As a talented group of professionals in our areas of specialization, we have formed a common goal to conquer any wound, regardless of the location, source, chronicity, or barriers to healing. At least, this may be our belief. Did any of us have a burning desire as aspiring professionals to work to advance the cause of evidence-based wound care? As a team of multidisciplinary professionals of physicians, nurses, pharmacists, physical therapists, researchers, and industry personnel, we were able to recognize an area of need for patients who suffer from chronic wounds. For each of us, there was something which ignited the passion to serve, to research, and to lend our expertise to this subject. Our strides to promote wound care from a multidisciplinary approach have led us to improved overall outcomes for our patients. Despite the vast amount of advanced wound care products available as well as an evidence-based practice that supports wet to dry dressings are substandard, I still receive daily calls from clinicians reporting new wound care orders for wet to dry dressings to be performed in the home setting, usually twice daily.
Despite the vast amount of advanced wound care products available as well as an evidence-based practice that supports wet to dry dressings are substandard, I still receive daily calls from clinicians reporting new wound care orders for wet to dry dressings to be performed in the home setting, usually twice daily. I am that person, the one who is thrilled the more challenging the wound looks. But I guess we all are, right? That is what has brought us together, this group of people who have never met, we are all connected through one simple thing, a passion for those hard to heal wounds. Unfortunately, we likely all have had those wounds that we have tried hard to heal and failed. That’s right, I have failed, we all have. I wish I was perfect; I wish I got it right from the start every time, but I don’t. It’s these really difficult wounds that help us grow as practitioners and I hope that as I share my mistakes with you, you don’t have to make them as well.
I am that person, the one who is thrilled the more challenging the wound looks. But I guess we all are, right? That is what has brought us together, this group of people who have never met, we are all connected through one simple thing, a passion for those hard to heal wounds. Unfortunately, we likely all have had those wounds that we have tried hard to heal and failed. That’s right, I have failed, we all have. I wish I was perfect; I wish I got it right from the start every time, but I don’t. It’s these really difficult wounds that help us grow as practitioners and I hope that as I share my mistakes with you, you don’t have to make them as well. Kim Thomas, DNP, is an Advanced Registered Nurse Practitioner and works at the University of Washington Health-Valley Medical Center. Kim has 20 years of experience in lower extremity wound care and nursing and has been an Advanced Practice Nurse since 2010. Her specialties include Wound, Ostomy, and Continence Nursing.
Kim Thomas, DNP, is an Advanced Registered Nurse Practitioner and works at the University of Washington Health-Valley Medical Center. Kim has 20 years of experience in lower extremity wound care and nursing and has been an Advanced Practice Nurse since 2010. Her specialties include Wound, Ostomy, and Continence Nursing.  James McKee, DPM, is a surgical podiatrist with MultiCare in Washington State where he works in a hospital-based clinic to facilitate limb-salvage in a high-risk population. Dr. McKee completed his residency in podiatric medicine and surgery (PMS-36) at Puget Sound VA in Seattle, Washington where he focused primarily on diabetic and limb-salvage medical and surgical treatments.
James McKee, DPM, is a surgical podiatrist with MultiCare in Washington State where he works in a hospital-based clinic to facilitate limb-salvage in a high-risk population. Dr. McKee completed his residency in podiatric medicine and surgery (PMS-36) at Puget Sound VA in Seattle, Washington where he focused primarily on diabetic and limb-salvage medical and surgical treatments. William Tettelbach, MD, is a certified wound specialist who is actively board certified in Undersea & Hyperbaric Medicine, Infectious Diseases, Internal Medicine with formal training in Biomedical Informatics. Dr. Tettelbach is currently the acting Associate Chief Medical Officer for MiMedx Group, Inc. and is the Medical Director of Wound Care, Antibiotic Stewardship, and Infection Prevention at Promise Hospital.
William Tettelbach, MD, is a certified wound specialist who is actively board certified in Undersea & Hyperbaric Medicine, Infectious Diseases, Internal Medicine with formal training in Biomedical Informatics. Dr. Tettelbach is currently the acting Associate Chief Medical Officer for MiMedx Group, Inc. and is the Medical Director of Wound Care, Antibiotic Stewardship, and Infection Prevention at Promise Hospital.
 “2020 is going to be a pivotal year for AAWC. Strong leadership is essential to carrying out a successful strategic plan. I am confident we have a highly proactive and experienced selection of qualified candidates. In the coming weeks, I encourage everyone to take the opportunity to get familiar with the Board of Directors candidates and vote.” Victoria E. Elliott, RPh, MBA, CAE, chief executive officer, Association for the Advancement of Wound Care.
“2020 is going to be a pivotal year for AAWC. Strong leadership is essential to carrying out a successful strategic plan. I am confident we have a highly proactive and experienced selection of qualified candidates. In the coming weeks, I encourage everyone to take the opportunity to get familiar with the Board of Directors candidates and vote.” Victoria E. Elliott, RPh, MBA, CAE, chief executive officer, Association for the Advancement of Wound Care. Members of AAWC are known for top tier commitment, specialization, and passion for wound care. Yet among the greatest of challenges advanced wound care professionals face, one is how to demonstrate and communicate the impact and value provided to administrators and clinicians managing or overseeing multiple clinical services.
Members of AAWC are known for top tier commitment, specialization, and passion for wound care. Yet among the greatest of challenges advanced wound care professionals face, one is how to demonstrate and communicate the impact and value provided to administrators and clinicians managing or overseeing multiple clinical services.

 doesn’t have all the tools, then the solution relies on creativity. Hands-on sessions encouraged attendees to be creative and get in the habit of choosing the best course of action given select resources when practicing wound care.
doesn’t have all the tools, then the solution relies on creativity. Hands-on sessions encouraged attendees to be creative and get in the habit of choosing the best course of action given select resources when practicing wound care. 



